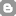COMPARISONS OF THE PROPERTIES OF SOLUTIONS, COLLOIDS AND SUSPENSIONS
| Property | Solutions | Colloids | Suspensions |
| Phase | Homogeneous | Heterogeneous but appear homogeneous to the naked eye | Heterogeneous |
| Particle Size | Less than 1nm | 1 to 100 nm | More than 100 nm |
| Appearance | Clear | Cloudy | Cloudy |
| Separation | Does not separate | Does not separate | Separates or settles |
| Scattering of Light (Tyndall Effect) | Does not Scatter | Scatters Light (Tyndall Effect) | Does not Scatter |
| Filterability | Cannot be Filtered | Cannot be Filtered | Can be Filtered |
| Examples | Salt solution, Air, vinegar, carbonated drinks *Alloys Bronze (copper and tin) Brass (copper and zinc) Steel (carbon and iron) | Cooked Starch, Smoke, fog, milk, chocolate, clouds, paint, gelatin, foam, marshmallow | Muddy water, Sand in water, arroz caldo |